Unexpected Anomaly Discovered On The Moon – Why Is Our Satellite Rusting?
Eddie Gonzales Jr. – MessageToEagle.com – Scientists have come across an unexpected anomaly on the Moon.
The Moon isn’t supposed to have oxygen or liquid water, so how can it be rusting?
Mars has long been known for its rust. Iron on its surface, combined with water and oxygen from the ancient past, give the Red Planet its hue. But scientists were recently surprised to find evidence that our airless Moon has rust on it as well.

Credit: Public Domain
In 2008, the Indian Space Research Organization’s Chandrayaan-1 orbiter surveyed the Moon’s surface and discovered water ice. But it is only recently researchers have discovered signatures in the data matching the iron oxide, hematite, a form of rust that normally requires the presence of both oxygen and water.
Lead author Shuai Li of the University of Hawaii has studied that water extensively in data from Chandrayaan-1’s Moon Mineralogy Mapper instrument, or M3, which was built by NASA’s Jet Propulsion Laboratory in Southern California and the results are surprising.
Water interacts with rock to produce a diversity of minerals, and M3 detected spectra – or light reflected off surfaces – that revealed the Moon’s poles had a very different composition than the rest of it.
“It’s very puzzling,” Li said. “The Moon is a terrible environment for hematite to form in.”
While the Moon’s surface is littered with iron-rich rocks, he nevertheless was surprised to find a close match with the spectral signature of hematite. The mineral is a form of iron oxide, or rust, produced when iron is exposed to oxygen and water. So, how can we explain the fact that the Moon is rusting if the satellite hasn’t any oxygen or liquid water?
Metal Mystery
The mystery starts with the solar wind, a stream of charged particles that flows out from the Sun, bombarding Earth and the Moon with hydrogen. Hydrogen makes it harder for hematite to form. It’s what is known as a reducer, meaning it adds electrons to the materials it interacts with.
That’s the opposite of what is needed to make hematite: For iron to rust, it requires an oxidizer, which removes electrons. And while the Earth has a magnetic field shielding it from this hydrogen, the Moon does not.
“It’s very puzzling,” Li said. “The Moon is a terrible environment for hematite to form in.” So, he turned to JPL scientists Abigail Fraeman and Vivian Sun to help poke at M3‘s data and confirm his discovery of hematite.
“At first, I totally didn’t believe it. It shouldn’t exist based on the conditions present on the Moon,” Fraeman said.
“But since we discovered water on the Moon, people have been speculating that there could be a greater variety of minerals than we realize if that water had reacted with rocks.”
The data does indeed indicate the presence of hematite at the lunar poles. “In the end, the spectra were convincingly hematite-bearing, and there needed to be an explanation for why it’s on the Moon,” Sun said.
3 Possible Explanations
Nothing is certain yet, but there are some possible explanations attempting to shed light on how rust might form in such an environment.
For starters, while the Moon lacks an atmosphere, it is in fact home to trace amounts of oxygen. The source of that oxygen: our planet. Earth’s magnetic field trails behind the planet like a windsock.
Then there’s the matter of all that hydrogen being delivered by the solar wind. As a reducer, hydrogen should prevent oxidation from occurring. But Earth’s magnetotail has a mediating effect. Besides ferrying oxygen to the Moon from our home planet, it also blocks over 99% of the solar wind during certain periods of the Moon’s orbit (specifically, whenever it’s in the full Moon phase). That opens occasional windows during the lunar cycle when rust can form.
The third piece of the puzzle is water. While most of the Moon is bone dry, water ice can be found in shadowed lunar craters on the Moon’s far side. But the hematite was detected far from that ice.
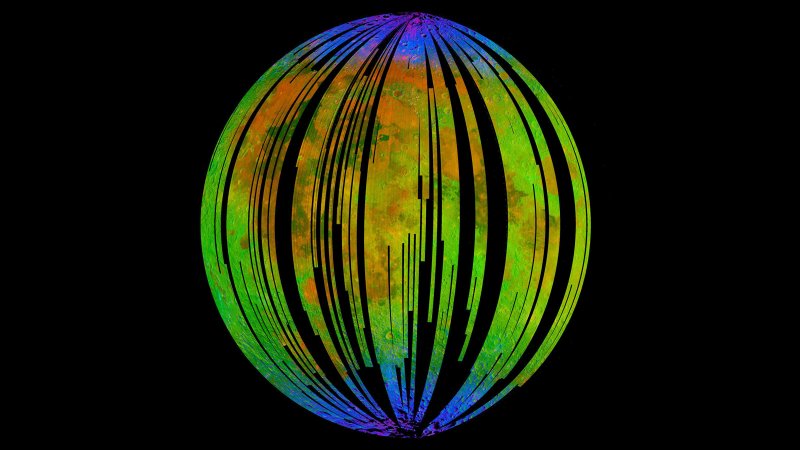
The blue areas in this composite image from the Moon Mineralogy Mapper (M3) aboard the Indian Space Research Organization’s Chandrayaan-1 orbiter show water concentrated at the Moon’s poles. Homing in on the spectra of rocks there, researcher found signs of hematite, a form of rust. Credit: ISRO/NASA/JPL-Caltech/Brown University/USGS
Li proposes that fast-moving dust particles that regularly pelt the Moon could release these surface-borne water molecules, mixing them with iron in the lunar soil. Heat from these impacts could increase the oxidation rate; the dust particles themselves may also be carrying water molecules, implanting them into the surface so that they mix with iron. During just the right moments – namely, when the Moon is shielded from the solar wind and oxygen is present – a rust-inducing chemical reaction could occur.
More data is needed to determine exactly how the water is interacting with rock. That data could also help explain another mystery: why smaller quantities of hematite are also forming on the far side of the Moon, where the Earth’s oxygen shouldn’t be able to reach it.
Written by Eddie Gonzales Jr. – MessageToEagle.com Staff