Unpredicted Discovery: Why Is Iron So Highly Oxidized Deep Inside The Earth? Geoscientists Are Surprised
MessageToEagle.com – Recent digging deep into the Earth’s interior revealed the highly oxidized iron—similar to the rust we see on our planet’s surface—within garnets found within diamonds.
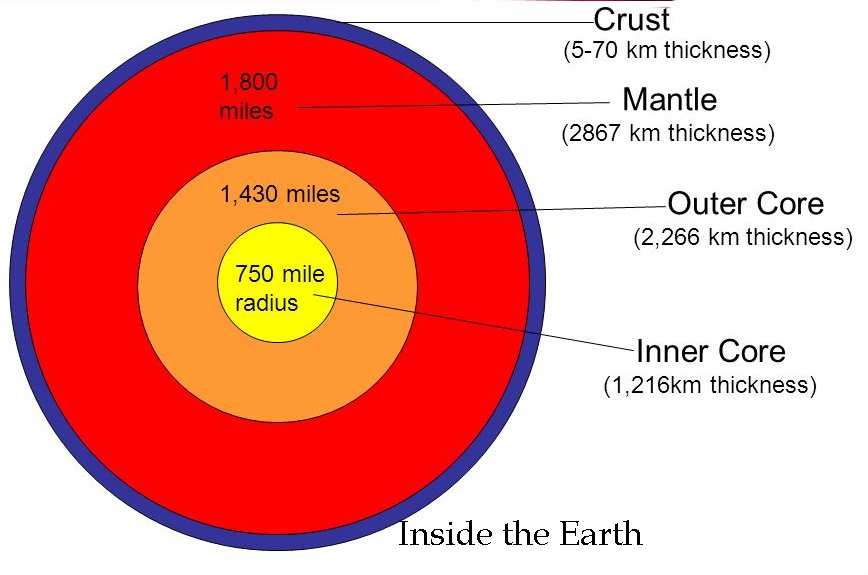
It is located five hundred fifty kilometers below the Earth’s surface.
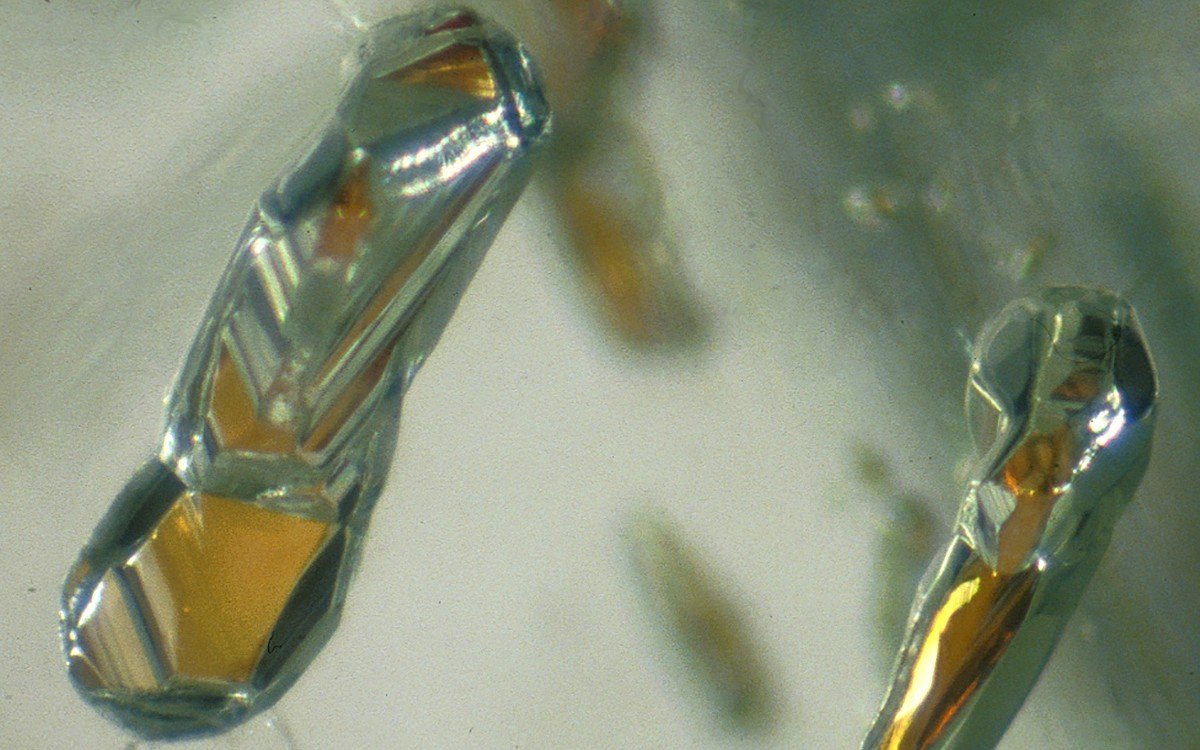
The result surprised geoscientists around the globe because there is little opportunity for iron to become so highly oxidized deep below the Earth’s surface.
“On Earth’s surface, where oxygen is plentiful, iron will oxidize to rust,” explained Thomas Stachel, professor in the Department of Earth and Atmospheric Sciences at the University of Alberta and co-author of the study.
“In the Earth’s deep mantle, we should find iron in its less oxidized form, known as ferrous iron, or in its metal form. But what we found was the exact opposite—the deeper we go, the more oxidized iron we found.”
The discovery suggests that something oxidized the rocks in which the super-deep diamonds were found.
The researchers believe that it was molten carbonate, carried to these great depths in sinking slabs of ancient sea floor.
The study suggests that surface carbon goes down as carbonates to at least 550 kilometers below the surface and there, the carbonates may melt and react with the surrounding rocks, eventually crystallizing into diamonds.
At the same time, the research helps to better understand the global carbon cycle that involves the transport of surface carbon back into the Earth’s mantle.
The study shows that the carbon cycle extends deep into mantle, possibly all the way down to the core-mantle boundary, with billion-year storage times.
The study was led by the University of Oxford with support from researchers in the Faculty of Science at the U of A.
Research is published in Nature Geoscience.
Original story- here.
MessageToEagle.com